Help to transform our extraordinary hospital into something even better.
- Refine search:
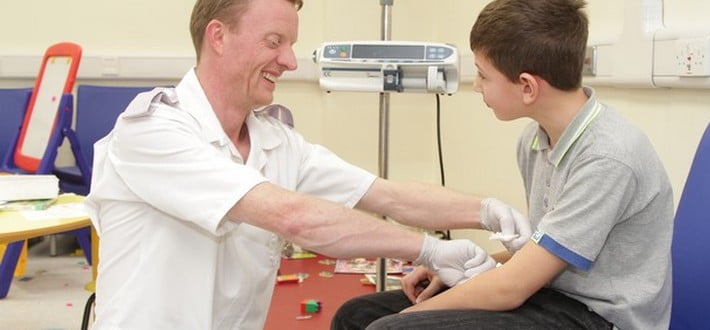
Every year we do research to improve the lives of thousands of children around the country. Research with children helps us understand more about disease and illness from a child’s perspective. It can also help us learn more about some adult diseases that begin in childhood.
Testing medicines is a significant area of research. Many medicines used with children have only been tested on adults, but the way their bodies handle medication can differ as can their reaction to diseases. It is important that further research is done to ensure that children are receiving the best treatment possible, whatever the severity of their illness.
See studies currently recruitingWe undertake several different types of clinical research:
You and your child might have to undertake various activities including:
Everything your child needs to do for the study will be explained to you by the research team and you will be given information leaflets to take home.
A member of the research team will explain the study to you including its purpose, any risks and what will be expected of you and your child if you decide to take part.
Once you have agreed to take part you will need to sign consent forms – even after you have signed these forms you can withdraw your child from the study at any time.
Remember that any involvement in research is voluntary and you don’t have to take part. It will not affect your child’s ongoing treatment if you refuse and you don’t have to give a reason for refusing. You might be asked why you decided not to take part to help the researchers find out if there is anything that they can change or improve in future.
If you would like to get involved in a research project or clinical trial, please contact the Clinical Research Facility team on 0114 271 7417. They will be happy to offer you practical advice on how your family can help.
By continuing to use the site, you agree to the use of cookies. more information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.