Help to transform our extraordinary hospital into something even better.
- Refine search:

11-year-old Henry was just getting to grips with what his Type 1 diabetes would mean for him and his family when a chance meeting brought him to Sheffield Children’s – and a place on a new research trial.
His mum Rachel said: “I’m a Dietitian and I was talking to a friend at work who mentioned that this trial was taking place. I contacted the team here because there was only a short window. The trial was specifically asking for newly diagnosed patients – it had to be within the first month. We just made it!”
Henry was originally diagnosed in June 2020 – when he was ten – at a local hospital in London, although Rachel had suspected something was wrong and carried out some home tests before taking Henry in to hospital.
Rachel said: “It was a devastating moment when we realised that our family would never be the same again.”
Type 1 diabetes causes the level of glucose in your blood to become too high. This happens because your body cannot produce enough of a hormone called insulin, which controls blood glucose. People with Type 1 diabetes usually need daily injections of insulin to keep their blood glucose levels under control.
This is what Henry began to do: “I learned how to do my insulin injections quite quickly,” he said.
Rachel added: “He was amazingly accepting of everything – he just took it in his stride.”
In August 2020, Henry started on the PROTECT trial, which uses the drug Teplizumab, here at Sheffield Children’s. Although many sites across the world have taken part in the trial only a few have been able to in the UK and Henry now travels up from London frequently for appointments.
The trial involved two courses of treatment in Sheffield with each lasting 12 consecutive days, six months apart. The drug is given intravenously, and participants are monitored closely. There are a lot of additional visits to hospital for the trial and tests including blood tests, questionnaires and 12/7 glucose monitoring.
Rachel said: “The trial is double blind, of course, so we have no way of knowing for sure that Henry has been receiving the drug. However, since Henry started the trial, we have seen an incredible impact on his life. Shortly after the first round of infusions, it became clear that Henry’s blood sugar levels had dropped to a level where insulin was no longer required – even on an ad hoc basis.
“Henry hasn’t had any insulin since August 2020 because the treatment has maintained whatever function he had – it’s been remarkable. You can only imagine the impact this has had on Henry’s physical and mental wellbeing – and the positive effect on our whole family’s lifestyle.”
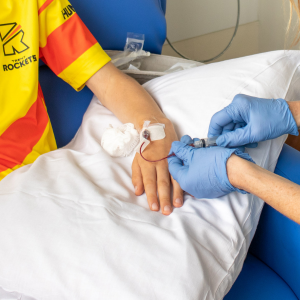 Although patients are expected to require insulin in the future it’s hoped that there are a number of short and long term benefits to preserving what is known as the ‘honeymoon period’ – which is when the body is still able to produce some insulin.
Although patients are expected to require insulin in the future it’s hoped that there are a number of short and long term benefits to preserving what is known as the ‘honeymoon period’ – which is when the body is still able to produce some insulin.
Since first arriving at Sheffield Children’s in August 2020, Henry and his family have got to know the team at the Clinical Research Facility (CRF).
Henry said: “They’re very good – they really know what they’re doing! They’ve made it easier for me to manage everything.”
Rachel added: “They remember a lot of little details and try to keep things as relaxed as possible. Everyone goes above and beyond – but especially Bee (a Research Nurse who has been with the family since their trial began).”
Bee said: ”It’s been lovely to get to know Henry and his family and it’s great to hear he’s enjoyed taking part in this intense trial. A type 1 diabetes diagnosis is a big shock to families and taking part in an intense trial isn’t for everyone especially so close to diagnosis. However, nine participants have taken part in this trial through Sheffield Children’s and many more were given the opportunity. The trial is now closed to recruitment internationally and we look forward to seeing the results of the trial in due course”.
Thank you for sharing your story Henry!
If you would like to find out more about participating in research at Sheffield Children’s, please visit our website: https://www.sheffieldchildrens.nhs.uk/research/take-part-in-research/
By continuing to use the site, you agree to the use of cookies. more information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.